DENSITOMETRY PROCEDURES
It is mandatory to present your medical prescription to be able to carry out the tests.
If you are a pregnant woman or if you suspect that you may be so, or if you are breast-feeding,
please notify it to our health staff before performing any test.
Densitometric tests do not involve any radioactive substance.
The access of companions to the center is allowed in the case of children and patients who require special attention.
The rest of the patients can come accompanied but, as a health precaution during the pandemic and depending on
the capacity of the waiting room and the criteria of our staff, it is possible that
the companion cannot wait inside the center.
DENSITOMETRY PROCEDURES
It is mandatory to present your medical prescription to be able to carry out the tests.
If you are a pregnant woman or if you suspect that you may be so, or if you are breast-feeding,
please notify it to our health staff before performing any test.
Densitometric tests do not involve any radioactive substance.
The access of companions to the center is allowed in the case of children and patients who require special attention.
The rest of the patients can come accompanied but, as a health precaution during the pandemic and depending on
the capacity of the waiting room and the criteria of our staff, it is possible that
the companion cannot wait inside the center.
abc
abc
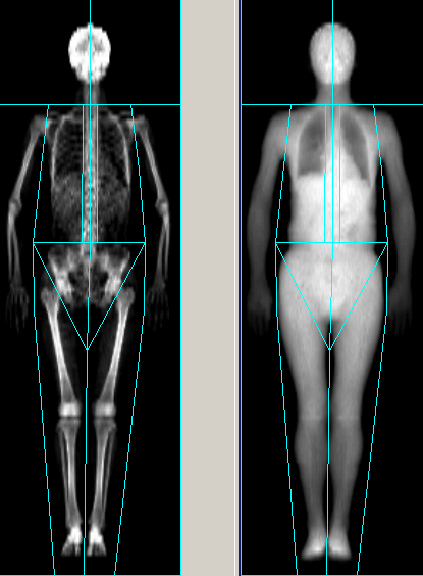
BODY COMPOSITION
DESCRIPTION: Measurement of body composition by dual energy X-ray absorptiometry, determining the different densities of soft tissues, on both upper and lower extremities, trunk, pelvic girdle and abdominal circumference, detailing the distribution of body fat, lean mass and bone skeleton. This distribution is important to evaluate diets, exercises and risks.
PREPARATION: No previous preparation needed.
INDICATIONS: Obesity, nervous anorexia, cystic fibrosis, HIV, chronic renal failure.
DURATION OF PROCEDURE: 20 minutes.
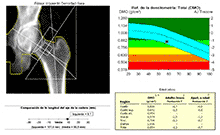
HIP PROSTHESIS DENSITOMETRY
DESCRIPTION: Evaluation of hip-prosthesis osteointegration by evaluating the mineral density of the bone adjacent to the prosthesis in selected periprosthetic regions.
PREPARATION: No previous preparation needed.
INDICATIONS: Evaluation of hip-prosthesis osteointegration.
DURATION OF PROCEDURE: 20 minuts.
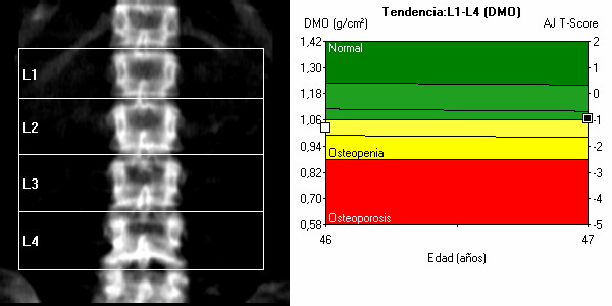
BONE DENSITOMETRY
DESCRIPTION: Evaluation of the degree of bone mineralization by means of bi-photonic absorptiometry of RX. Morphometry studies.
Normal densitometry is done in the femur (hip) and lumbar spine (vertebral bodies L1 to L4). The determining value is the lowest of both sites.
If at least two contiguous vertebrae can not be analyzed or if there are vertebrae with fracture sequelae or focal lesion and/or those that have more than one standard-deviation difference with the rest of the segment, the lumbar study is not appraisable. Hips with fracture sequelae, focal lesions or prostheses should also be discarded.
Other conditions that make the measurement of bone density not feasible in the lumbar spine or hip are neurological deficits, pain or obesity of the patient. In these cases, it is possible to replace normal densitometry with forearm densitometry, usually performed in the non-dominant forearm, that is, the left will be studied if the patient is right-handed and vice versa.
PREPARATION: No previous preparation needed.
INDICATIONS: Existence of risk factors for osteoporosis (hyperthyroidism, early menopause [<45 years] in order to diagnose significant bone demineralizations to help make therapeutic decisions, prolonged secondary amenorrhea, primary hypogonadism, patients undergoing long-term corticosteroid therapy (<7.5 mg/day, > one year).
Conditions associated with osteoporosis (primary hyperparathyroidism, nervous anorexia, under-absorption syndromes, post-transplants, chronic renal failure, imperfect osteogenesis, neoplasms, prolonged immobilization, Cushing’s syndrome).
Radiological evidence of osteopenia and/or vertebral deformities.
Previous fractures due to fragility (hip, spine, wrist or proximal humerus).
Significant height loss or marked kyphosis.
Therapeutic monitoring (treatment with bi-phosphonates, calcitonin, fluorinated salts, D-vitamin; hormone replacement therapy in patients with secondary osteoporosis).
DURATION OF PROCEDURE: 20 minutes.
BODY COMPOSITION
DESCRIPTION
Measurement of body composition by dual energy X-ray absorptiometry, determining the different densities of soft tissues, on both upper and lower extremities, trunk, pelvic girdle and abdominal circumference, detailing the distribution of body fat, lean mass and bone skeleton. This distribution is important to evaluate diets, exercises and risks.
PREPARATION
No previous preparation needed.
INDICATIONS
- Obesity
- Nervous anorexia
- Cystic fibrosis
- AIDS
- Chronic renal insufficiency
DURATION OF PROCEDURE
20 minutes.
HIP-PROSTHESIS DENSITOMETRY
DESCRIPTION
Evaluation of hip-prosthesis osteointegration by evaluating the mineral density of the bone adjacent to the prosthesis in selected periprosthetic regions.
PREPARATION
No previous preparation needed.
INDICATIONS
Evaluation of hip-prosthesis osteointegration.
DURATION OF PROCEDURE
20 minutes.
BONE DENSITOMETRY
(LUMBAR COLUMN, FEMALE, FOREARM)
DESCRIPTION
Evaluation of the degree of bone mineralization by means of bi-photonic absorptiometry of RX. Morphometry studies.
Normal densitometry is done in the femur (hip) and lumbar spine (vertebral bodies L1 to L4). The determining value is the lowest of both sites.
If at least two contiguous vertebrae can not be analyzed or if there are vertebrae with fracture sequelae or focal lesion and/or those that have more than one standard-deviation difference with the rest of the segment, the lumbar study is not appraisable. Hips with fracture sequelae, focal lesions or prostheses should also be discarded.
Other conditions that make the measurement of bone density not feasible in the lumbar spine or hip are neurological deficits, pain or obesity of the patient. In these cases, it is possible to replace normal densitometry with forearm densitometry, usually performed in the non-dominant forearm, that is, the left will be studied if the patient is right-handed and vice versa.
PREPARATION
No previous preparation needed.
INDICATIONS
- Existence of risk factors for osteoporosis
- Hyperthyroidism
- Early menopause [<45 years], in order to diagnose significant bone demineralizations to help make therapeutic decisions
- Prolonged secondary amenorrhea
- Primary hypogonadism
- Patients undergoing long-term corticosteroid therapy (<7.5 mg/day, > one year).
- Conditions associated with osteoporosis
- Primary hyperparathyroidism
- Nervous anorexia
- Under-absorption syndromes
- Post-transplants
- Chronic renal failure
- Imperfect osteogenesis
- Neoplasms
- Prolonged immobilization
- Cushing’s syndrome.
- Radiological evidence of osteopenia and/or vertebral deformities.
- Previous fractures due to fragility (hip, spine, wrist or proximal humerus).
- Significant height loss or marked kyphosis.
- Therapeutic monitoring
- Treatment with bi-phosphonates, calcitonin, fluorinated salts or D-vitamin
- Hormone replacement therapy in patients with secondary osteoporosis.
DURATION OF PROCEDURE
20 minutes.

ATRYS-SIMM IRLA
Josep Irla i Bosch Street, 5, Building 2
Barcelona 08034, Catalonia, SPAIN
Phone (+34) 93 204 6439
ATRYS-SIMM SJD
Santa Rosa Street 39, Pediatric Cancer Center Barcelona
Esplugues de Llobregat 08950, Catalonia, SPAIN
Phone (+34) 93 254 0470
FAX: (+34) 93 204 9641 · E-MAIL: info.simm@atryshealth.com
At ATRYS-SIMM your personal data is treated in accordance with the principles and rights contained in the GDPR law 2016/679 of April 27, 2016 and in the LOPDGDD law 3/2018 of December 5, 2018.
You can exercise your rights by contacting us by email to protecciondatos-sp@atryshealth.com.
ATRYS-SIMM IRLA
Josep Irla i Bosch Street, 5, Building 2
Barcelona 08034, Catalonia, SPAIN
Phone (+34) 93 204 6439
ATRYS-SIMM SJD
Santa Rosa Street, 39
Esplugues de Llobregat 08950, Catalonia, SPAIN
Phone (+34) 93 254 0470
FAX: (+34) 93 204 9641
E-MAIL: info.simm@atryshealth.com
At ATRYS-SIMM your personal data is treated in accordance with
the principles and rights contained in the GDPR law 2016/679 of
April 27, 2016 and in the LOPDGDD law 3/2018 of December 5, 2018.
You can exercise your rights by contacting us by
email to protecciondatos-sp@atryshealth.com.


