OSTEOARTICULAR PATHOLOGY PROCEDURES
It is mandatory to present your medical prescription to be able to carry out the tests.
If you are a pregnant woman or if you suspect that you may be so, or if you are breast-feeding,
please notify it to our health staff before performing any test.
All nuclear medicine tests involve the administration of a radiopharmaceutical
with a very low dose of radioactivity, which is eliminated in the following hours through the urinary or digestive routes.
The access of companions to the center is allowed in the case of children and patients who require special attention.
The rest of the patients can come accompanied but, as a health precaution during the pandemic and depending on
the capacity of the waiting room and the criteria of our staff, it is possible that
the companion cannot wait inside the center.
OSTEOARTICULAR PATHOLOGY
PROCEDURES
It is mandatory to present your medical prescription to be able to carry out the tests.
If you are a pregnant woman or if you suspect that you may be so, or if you are breast-feeding,
please notify it to our health staff before performing any test.
All nuclear medicine tests involve the administration of a radiopharmaceutical
with a very low dose of radioactivity, which is eliminated in the following hours through the urinary or digestive routes.
The access of companions to the center is allowed in the case of children and patients who require special attention.
The rest of the patients can come accompanied but, as a health precaution during the pandemic and depending on
the capacity of the waiting room and the criteria of our staff, it is possible that
the companion cannot wait inside the center.
abc
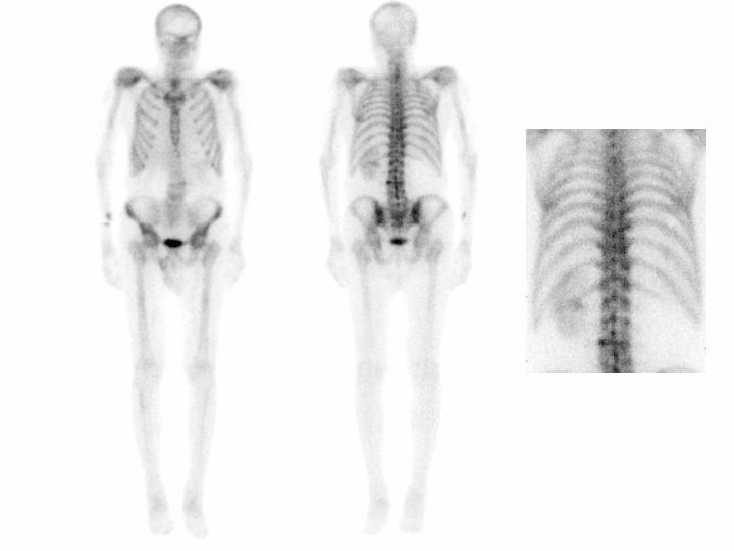
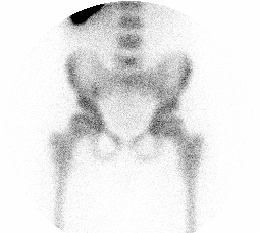
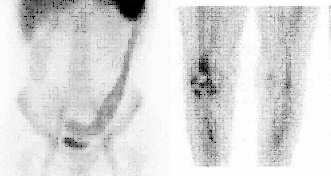
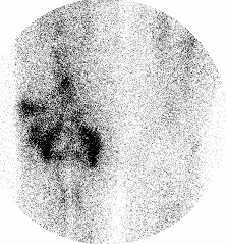
BONE SCINTIGRAPHY
BONE SPECT-CT WITH FUSION
BONE TOMOSCINTIGRAPHY (SPET/SPET-CT)
DESCRIPTION: Evaluation of bone metabolism and affections implying the skeleton. Detection of small-size or uncertain-location bone injuries difficult to evaluate in planar images (bone SPET: 33-197-PD 18).
PREPARATION: No previous preparation is needed. No fasting required.
INDICATIONS: Detection of bone metastases. Assessment of primitive bone tumors. Bone repercussion of bone marrow alterations. Evaluation of joint and osteoarticular affectations (inflammatory arthropathy, infections or degenerative diseases). Metabolic bone disease. Evaluation of musculoskeletal traumatic pathologies and sports injuries. Evaluation of joint prostheses and bone grafts. Evaluation of bone recovery in vascular disease (avascular bone necrosis, algodistrophy).
DURATION OF PROCEDURE: 3 hours.
MACROPHAGIC BONE-MARROW SCINTIGRAPHY
DESCRIPTION: Evaluation of the distribution of the reticuloendothelial system at bone marrow, liver and spleen level.
PREPARATION: No preparation is needed.
INDICATIONS: Evaluation of the distribution of normal bone marrow in cases of endometrial prosthesis, osteosynthesis or spinal cord infiltration, as a complement of bone or labeled leukocytes scintigraphy.
DURATION OF PROCEDURE: 2 hours.
LABELED LEUKOCYTES SCINTIGRAPHY FOR
THE STUDY OF BONE INFECTIONS
LABELED LEUKOCYTES SCINTIGRAPHIC SCAN
FOR THE STUDY OF BONE INFECTIONS
LABELED LEUKOCYTES SPECT-TC
TOMOGRAPHY FOR THE STUDY OF
BONE INFECTIONS
DESCRIPTION: Evaluation of the distribution of labeled leukocytes in inflammatory and infectious processes.
PREPARATION: 6-hour minimum fasting is recommended.
INDICATIONS: Detection and localization of bone infections (abscesses at any location, osteoarticular, osteosynthesis or joint prostheses, vascular prostheses). Diagnosis of fever of unknown origin.
DURATION OF PROCEDURE: 4 to 8 hours. The labeling process is lengthy (4 hours). Images must be made after a waiting period (30 minutes for early images and 2 to 6 hours for late images).
abc
MONOCLONAL ANTIBODIES SCINTIGRAPHY
IMMUNOSCINTIGRAPHIC SCAN
MONOCLONAL ANTIBODIES SCINTIGRAPHIC SCAN
DESCRIPTION: Evaluation of the distribution of granulocytes labeled with antigranulocytic antibodies in bone and osteoarthritic infectious processes.
PREPARATION: No preparation is needed.
INDICATIONS: Evaluation of bone and osteoarticular infectious processes.
DURATION OF PROCEDURE: 4 hours.
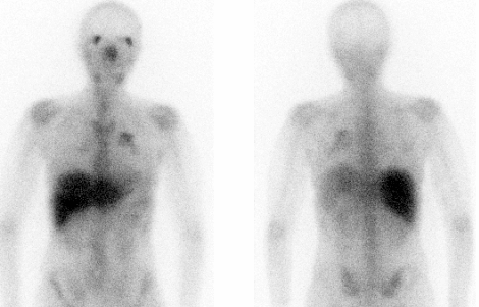
Ga-67 BONE SCINTIGRAPHY
Ga-67 FUSION BONE SPECT-CT
Ga-67 BONE TOMOSCINTIGRAPHY (SPET/SPET-CT)
DESCRIPTION: Evaluation of inflammatory, infectious or tumoral activity.
PREPARATION: When the detection involves abdominal organs, the use of laxatives is recommended, to reduce the traffic to the colon. It is advisable to suppress iron (Fe) administration 48 hours before the radiopharmaceutical administration and up to 48 hours afterward. As long as the procedure is in progress, the administration of gadolinium should be avoided if an MR is practiced.
INDICATIONS: Evaluation of patients with Hodgkin’s disease and non-Hodgkin lymphomas, both in the assessment of pretreatment tumor uptake, control of therapeutic response (differential diagnosis of tumor activity versus residual mass), detection of recurrences and evaluation of the extent of the disease. Detection and localization of other tumors (sarcomas). Evaluation of AIDS patients. Evaluation of sarcoidosis, fibrosis and chronic infections (chronic osteomyelitis). Diagnosis and localization of pulmonary infections. Evaluation of pulmonary interstitial diseases (specifically sarcoidosis and fibrosis).
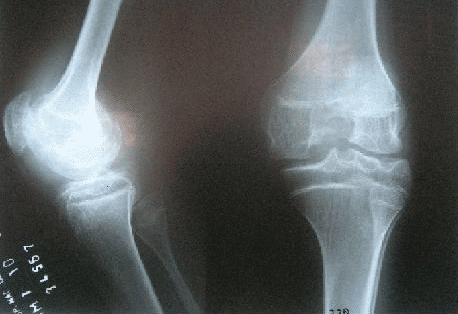
TREATMENT OF SYNOVITIS IN CHRONIC INFLAMMATORY ARTHROPATHIES
RADIOISOTOPIC ENDOCAVITARY TREATMENT
DESCRIPTION: Treatment of synovitis in chronic inflammatory arthropathies.
PREPARATION: The treatment is done after consultation with the prescribing doctor (rheumatologist, traumatologist, systemic illnesses specialist, etc.) and once he/she has established that there is no local contraindication (supported by X-rays, MR or CT if necessary).
INDICATIONS: Treatment of the synovitis of chronic inflammatory arthropathies (rheumatoid arthritis, other treatment-resistant arthropathies such as seronegative arthropathy, hemophilia, villonodular synovitis). Assessing the possibility of treating chronic degenerative arthropathy.
DURATION OF PROCEDURE: 30 minutes.
abc
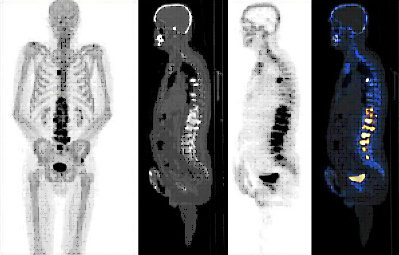
18F-SODIUM FLUORIDE (18F-NaF) PET-CT
DESCRIPTION: Study of bone metabolism.
PREPARATION: No previous preparation is needed. No fasting required.
INDICATIONS: Study of primary bone tumors, bone viability, bone necrosis and other benign bone disorders. Bone staging of prostate cancer, breast cancer and other neoplastic diseases with blastic bone metastases.
DURATION OF PROCEDURE: 1 hour.
BONE SCINTIGRAPHY
BONE SPECT-CT WITH FUSION
BONE TOMOSCINTIGRAPHY (SPET/SPET-CT)
DESCRIPTION
- Evaluation of bone metabolism and affections implying the skeleton.
- Detection of small-size or uncertain-location bone injuries difficult to evaluate in planar images (bone SPET: 33-197-PD 18).
PREPARATION
- No previous preparation is needed.
- No fasting required.
INDICATIONS
- Detection of bone metastases.
- Assessment of primitive bone tumors.
- Bone repercussion of bone marrow alterations.
- Evaluation of joint and osteoarticular affectations (inflammatory arthropathy, infections or degenerative diseases).
- Metabolic bone disease.
- Evaluation of musculoskeletal traumatic pathologies and sports injuries.
- Evaluation of joint prostheses and bone grafts.
- Evaluation of bone recovery in vascular disease (avascular bone necrosis, algodistrophy).
DURATION OF PROCEDURE
3 hours.
MACROPHAGIC BONE-MARROW SCINTIGRAPHY
DESCRIPTION
Evaluation of the distribution of the reticuloendothelial system at bone marrow, liver and spleen level.
PREPARATION
No preparation is needed.
INDICATIONS
Evaluation of normal bone marrow distribution in cases of endometrial prosthesis, osteosynthesis or spinal cord infiltration, as a complement of bone or labeled leukocytes scintigraphy.
DURATION OF PROCEDURE
DURATION OF PROCEDURE
2 hours.
LABELED LEUKOCYTES SCINTIGRAPHY FOR
THE STUDY OF BONE INFECTIONS
LABELED LEUKOCYTES SCINTIGRAPHIC SCAN
FOR THE STUDY OF BONE INFECTIONS
LABELED LEUKOCYTES SPECT-TC
TOMOGRAPHY FOR THE STUDY OF
BONE INFECTIONS
DESCRIPTION
Evaluation of the distribution of labeled leukocytes in inflammatory and infectious processes.
PREPARATION
Se recomienda un ayuno mínimo de 6 horas.
INDICATIONS
- Detection and localization of bone infections:
- Abscesses at any location
- Osteoarticular
- Osteosynthesis or joint prostheses
- Vascular prostheses).
- Diagnosis of fever of unknown origin.
DURATION OF PROCEDURE
4 a 8 horas. El proceso de marcado es largo (4 horas). Las imágenes han de 4 to 8 hours. The labeling process is lengthy (4 hours). Images must be made after a waiting period (30 minutes for early images and 2 to 6 hours for late images).
MONOCLONAL ANTIBODIES SCINTIGRAPHY
IMMUNOSCINTIGRAPHIC SCAN
MONOCLONAL ANTIBODIES
SCINTIGRAPHIC SCAN
DESCRIPTION
Evaluation of the distribution of granulocytes labeled with antigranulocytic antibodies in bone and osteoarthritic infectious processes.
PREPARATION
No preparation is needed.
INDICATIONS
Evaluation of bone and osteoarticular infectious processes.
DURATION OF PROCEDURE
4 hours.
Ga-67 BONE SCINTIGRAPHY
Ga-67 FUSION BONE SPECT-CT
Ga-67 BONE TOMOSCINTIGRAPHY
(SPET/SPET-CT)
DESCRIPTION
Evaluation of inflammatory, infectious or tumoral activity.
PREPARATION
-
When the detection involves abdominal organs, the use of laxatives is recommended, to reduce the traffic to the colon.
-
It is advisable to suppress iron (Fe) administration 48 hours before the radiopharmaceutical administration and up to 48 hours afterward.
-
As long as the procedure is in progress, the administration of gadolinium should be avoided if an MR is practiced.
INDICATIONS
- Evaluation of patients with Hodgkin’s disease and non-Hodgkin lymphomas, both in the assessment of pretreatment tumor uptake, control of therapeutic response (differential diagnosis of tumor activity versus residual mass), detection of recurrences and evaluation of the extent of the disease.
- Detection and localization of other tumors (sarcomas).
- Evaluation of AIDS patients.
- Evaluation of sarcoidosis, fibrosis and chronic infections (chronic osteomyelitis).
- Diagnosis and localization of pulmonary infections.
- Evaluation of pulmonary interstitial diseases (specifically sarcoidosis and fibrosis).
TREATMENT OF SYNOVITIS IN CHRONIC
INFLAMMATORY ARTHROPATHIES
RADIOISOTOPIC ENDOCAVITARY TREATMENT
DESCRIPTION
Treatment of synovitis in chronic inflammatory arthropathies.
PREPARATION
The treatment is done after consultation with the prescribing doctor (rheumatologist, traumatologist, systemic illnesses specialist, etc.) and once he/she has established that there is no local contraindication (supported by X-rays, MR or CT if necessary).
INDICATIONS
- Treatment of the synovitis of chronic inflammatory arthropathies:
- Rheumatoid arthritis
- Other treatment-resistant arthropathies such as seronegative arthropathy, hemophilia, villonodular synovitis.
- Assessing the possibility of treating chronic degenerative arthropathy.
DURATION OF PROCEDURE
30 minutes.
18F-SODIUM FLUORIDE (18F-NaF) PET-CT
DESCRIPTION
Study of bone metabolism.
PREPARATION
- No previous preparation is needed.
- No fasting required.
INDICATIONS
- Study of:
- Primary bone tumors.
- Bone viability.
- Bone necrosis.
- Other benign bone disorders.
- Bone staging of:
- Prostate cancer.
- Breast cancer.
- Other neoplastic diseases with blastic bone metastases.
DURATION OF PROCEDURE
1 hour.
.

SIMM MOLECULAR, S.L.U. belongs to the ATRYS Group
ATRYS-SIMM IRLA Center
Josep Irla i Bosch Street, 5, Buildong 2
Barcelona 08034, Catalonia, SPAIN
Phone (+34) 93 204 6439
ATRYS-SIMM SJD Center
Santa Rosa Street, 39, Pediatric Cancer Center Barcelona
Esplugues de Llobregat 08950, Catalonia, SPAIN
Phone (+34) 93 254 0470
FAX: (+34) 93 204 9641 · EMAIL: info.simm@atryshealth.com
In ATRYS-SIMM your personal data is treated according to the principles and rights included in the Spanish laws GDPR 2016/679 of April 27th, 2016 and LOPDGDD 3/2018 of December 5th, 2018.
You can excercise your rights by contacting us by email to protecciondatos-sp@atryshealth.com.
SIMM MOLECULAR, S.L.U. belons to the ATRYS Group
ATRYS-SIMM IRLA
Josep Irla i Bosch Street, 5, Building 2
Barcelona 08034, Catalonia, SPAIN
Phone (+34) 93 204 6439
ATRYS-SIMM SJD
Santa Rosa Street, 39
Esplugues de Llobregat 08950, Catalonia, SPAIN
Phone (+34) 93 254 0470
FAX: (+34) 93 204 9641
E-MAIL: info.simm@atryshealth.com
At ATRYS-SIMM your personal data is treated in accordance with
the principles and rights contained in the GDPR law 2016/679 of
April 27, 2016 and in the LOPDGDD law 3/2018 of December 5, 2018.
You can exercise your rights by contacting us by
email to protecciondatos-sp@atryshealth.com.