ONCOLOGY PROCEDURES
It is mandatory to present your medical prescription to be able to carry out the tests.
If you are a pregnant woman or if you suspect that you may be so, or if you are breast-feeding,
please notify it to our health staff before performing any test.
All nuclear medicine tests involve the administration of a radiopharmaceutical
with a very low dose of radioactivity, which is eliminated in the following hours through the urinary or digestive routes.
The access of companions to the center is allowed in the case of children and patients who require special attention.
The rest of the patients can come accompanied but, as a health precaution during the pandemic and depending on
the capacity of the waiting room and the criteria of our staff, it is possible that
the companion cannot wait inside the center.
ONCOLOGY PROCEDURES
It is mandatory to present your medical prescription to be able to carry out the tests.
If you are a pregnant woman or if you suspect that you may be so, or if you are breast-feeding,
please notify it to our health staff before performing any test.
All nuclear medicine tests involve the administration of a radiopharmaceutical
with a very low dose of radioactivity, which is eliminated in the following hours through the urinary or digestive routes.
The access of companions to the center is allowed in the case of children and patients who require special attention.
The rest of the patients can come accompanied but, as a health precaution during the pandemic and depending on
the capacity of the waiting room and the criteria of our staff, it is possible that
the companion cannot wait inside the center.
abc
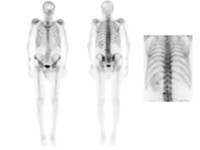
BONE SCINTIGRAPHY
BONE SPECT-CT WITH FUSION
BONE TOMOSCINTIGRAPHY (SPET/SPET-CT)
DESCRIPTION: Evaluation of bone metabolism and affections implying the skeleton. Detection of small-size or uncertain-location bone injuries difficult to evaluate in planar images (bone SPET: 33-197-PD 18).
PREPARATION: No previous preparation is needed. No fasting required.
INDICATIONS: Detection of bone metastases. Assessment of primitive bone tumors. Bone repercussion of bone marrow alterations. Evaluation of joint and osteoarticular affectations (inflammatory arthropathy, infections or degenerative diseases). Metabolic bone disease. Evaluation of musculoskeletal traumatic pathologies and sports injuries. Evaluation of joint prostheses and bone grafts. Evaluation of bone recovery in vascular disease (avascular bone necrosis, algodistrophy).
DURATION OF PROCEDURE: 3 hours.
BREAST SCINTIGRAPHY
DESCRIPTION: Evaluation of the existence, extension and staging of breast cancer.
PREPARATION: No previous preparation is needed.
INDICATIONS: Diagnosis of breast tumors. Evaluation of axillary lymph node involvement.
DURATION OF PROCEDURE: 1 hour.
META-IODO-BENZYL-GUANIDINE (MIBG) SCINTIGRAPHY
META-IODO-BENZYL-GUANIDINE (MIBG) SCINTIGRAPHIC SCAN
META-IODO-BENZYL-GUANIDINE (MIBG) TOMOSCINTIGRAPHY (SPET/SPET-CT)
DESCRIPTION: Evaluation of the existence of metabolic activity of the catecholamines pathway at the adrenal level and in neuroendocrine tumors.
PREPARATION: The thyroid gland must be stunned with 20 drops of potassium iodide 45-60 minutes before the I-123 tracer injection. This preventive treatment must be started 5 days before the administration of the dose and must extend until 3 days after the end of the procedure if it is done with I-131.
INDICATIONS: Identification, location and evaluation of the extension of tumors of neuroectodermal tissue: poisonous or malignant pheochromocytoma of intra or extra-adrenal localization, neuroblastomes, carcinoid tumors, medular thyroid carcinoma, paragangliomes, multiple endocrine neoplasies (MEN IIa and MEN IIb).
DURATION OF PROCEDURE: 2 days.
abc
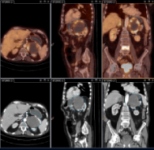
ONCOLOGIC PET-CT WITH 18F-FDG
DESCRIPTION: Study of oncologic patients.
PREPARATION: Fasting of at least 6 hours before performing the procedure is required. In general, it is advisable that patients take a light meal the night before the study and that they do not ingest anything until after it has been done. On the day of the procedure, the patient will have to drink abundant water (0.5-1L). Juices, sugary solutions, sweets etc. are not allowed. Urine must not be retained. Intravenous glucose solutions should also interrupt at least 6 hours before the scheduled time of the procedure. The patient should take his/her usual medication. It is necessary to avoid doing physical exercises 2-3 days before the procedure since this could condition a high muscular uptake of the tracer. Diabetic type II and hyperglycemic patients treated with oral antidiabetic medicines should take their usual medication early in the morning and preferably stay in bed at night before performing the test. Type I or diabetic patients with subcutaneous/intravenous insulin therapy should take their usual medication early in the morning. To avoid possible hypoglycemia, these patients can take some light breakfast, with a minimum of 4 hours before the procedure.
INDICATIONS: Characterization of pancreatic masses. Detection of lung pulmonary nodules and evident tumors of unknown origin. Detection of recurrences of gliomas with high degree of malignancy, head and neck tumors, differential thyroid cancer (high TG and negative RCI), primary lung, breast, pancreatic, colorectal and other carcinomas, ovarian cancer, malignant lymphomas, malignant melanomas. Staging of malignant melanoma, primary lung cancer, locally advanced breast, aesophagus, pancreatic and colorectal cancer, head and neck tumors, malignant lymphomas. Monitoring of the response to the treatment of malignant lymphoma and head and neck tumors.
DURATION OF PROCEDURE: 2 to 3 hours.
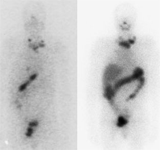
SCAN FOR THE DETECTION OF
THYROID METASTASES
I-123 SCINTIGRAPHIC SCAN
(WITH OR WITHOUT rhTSH)
I-131 SCINTIGRAPHIC SCAN
(WITH OR WITHOUT rhTSH)
I-123 TOMOSCINTIGRAPHY
(SPET/SPET-CT)
DESCRIPTION: Evaluation of the metabolic activity of thyroid tissue remnants of differentiated thyroid neoplasms.
PREPARATION: The patient must suspend his/her hormonal treatment (thyroxin [T4] for at least 21 days, triyodothyronine [T3] for at least 10 days). If hormonal suppression is not advised, TSH can be stimulated with two intramuscular injections in two consecutive days of rhTSH (Thyrogen). The patient must not be in iodic saturation, either because of intravenous or intrathecal scans with iodized contrast (skinography, TAC with contrast, myelography and some angiographies) at least 3 weeks before the procedure, or because of treatment with amniodarone at least 6 months before the procedure.
INDICATIONS: Evaluation of the existence of functioning thyroid tissue remnants after a thyroidectomy. Study of the spread of differentiated thyroid carcinoma. Detection of recurrences of differentiated thyroid carcinoma.
DURATION OF PROCEDURE: 3 days.
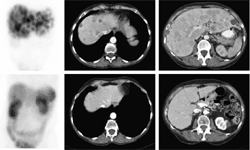
SCINTIGRAPHY WITH
SOMATOSTATIN RECEPTORS
SCINTIGRAPHY-SPECT-CT WITH 111In
OR 99mTc-OCTREOTIDE
SCINTIGRAPHIC SCAN WITH
SOMATOSTATIN RECEPTORS
TOMOGRAPHY-SPECT-CT WITH
SOMATOSTATIN RECEPTORS
DESCRIPTION: Evaluation of somatostatin receptor activity in tumors.
PREPARATION: No scans with barium or iodine contrast should be performed during the procedure. For the study of abdominal tumors, it is advisable to use laxatives, and even the use of enemas.
INDICATIONS: Evaluation of tumors that express somatostatin receptors (extension study, follow-up, control of therapeutic response and recurrence detection) in neuroendocrine tumors, such as carcinoid, enteropancreatic tumors and tumors derived from the neural crest (medullary of thyroid, neuroblastomas, paragangliomas and pheochromocytomas). Evaluation of other types of neoplasms that may have expression of somatostatin receptors, such as breast cancer, lung cancer, lymphomas and thyroid cancer.
DURATION OF PROCEDURE: 1 to 2 days.
abc
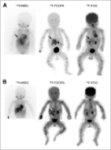
18F-DOPA PET-TC
DESCRIPTION: Evaluation of somatostatin receptor activity in tumors.
PREPARATION: No scans with barium or iodine contrast should be performed during the procedure. For the study of abdominal tumors, it is advisable to use laxatives, and even the use of enemas.
INDICATIONS: Evaluation of tumors that express somatostatin receptors (extension study, follow-up, control of therapeutic response and recurrence detection) in neuroendocrine tumors, such as carcinoid, enteropancreatic tumors and tumors derived from the neural crest (medullary of thyroid, neuroblastomas, paragangliomas and pheochromocytomas). Evaluation of other types of neoplasms that may have expression of somatostatin receptors, such as breast cancer, lung cancer, lymphomas and thyroid cancer.
DURATION OF PROCEDURE: 1 to 2 days.
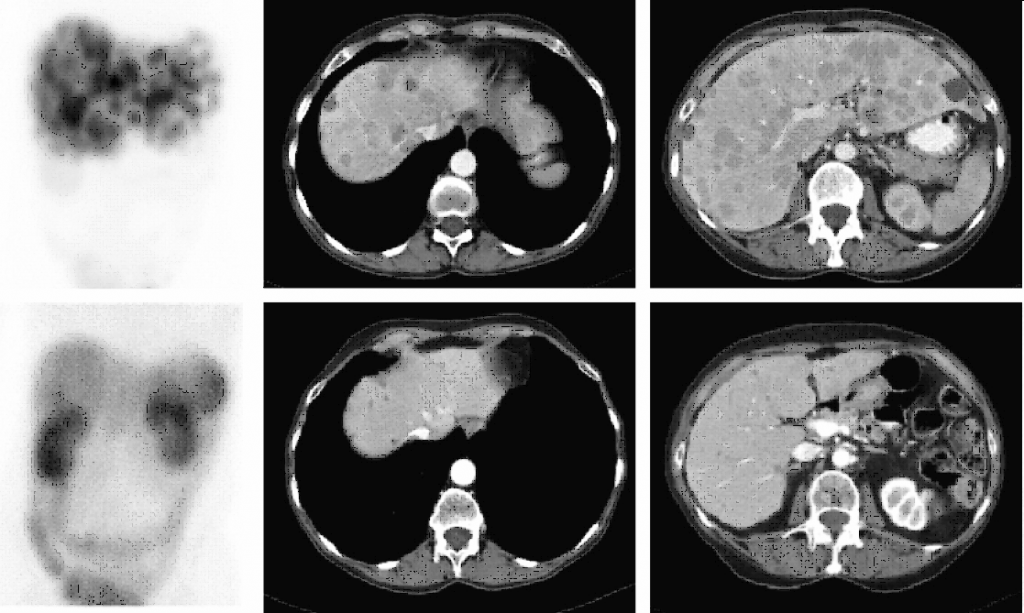
68Ga-DOTATOC PET-TC
DESCRIPTION: Evaluation of somatostatin receptor activity in tumors.
PREPARATION: No scans with barium or iodine contrast should be performed during the procedure. For the study of abdominal tumors, it is advisable to use laxatives, and even the use of enemas.
INDICATIONS: Evaluation of tumors that express somatostatin receptors (extension study, follow-up, control of therapeutic response and recurrence detection) in neuroendocrine tumors, such as carcinoid, enteropancreatic tumors and tumors derived from the neural crest (medullary of thyroid, neuroblastomas, paragangliomas and pheochromocytomas). Evaluation of other types of neoplasms that may have expression of somatostatin receptors, such as breast cancer, lung cancer, lymphomas and thyroid cancer.
DURATION OF PROCEDURE: 1 to 2 days.
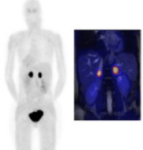
PSMA PET-TC
DESCRIPTION: Functional and anatomical body assessment of prostate cancer patients through the study of cell membrane synthesis.
PREPARATION: No prior preparation needed. No fasting is required.
INDICATIONS: Prostate cancer staging and follow-up.
DURATION OF PROCEDURE: 1 hour.
abc
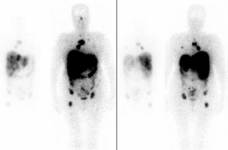
SCINTIGRAPHIC SCAN WITH TUMOR-AFFINITY OR METABOLIC-ACTIVITY TRACERS
(THALLIUM CHLORIDE, 99mTc-MIBI)
TOMOSCINTIGRAPHY (SPET/SPET-CT) WITH TUMOR-AFFINITY OR METABOLIC-ACTIVITY TRACERS
DESCRIPTION: Evaluation of metabolic activity in tumors.
PREPARATION: No previous preparation is needed.
INDICATIONS: Evaluation of tumoral activity in general (pulmonary, muscular, breast, brain, etc.).
DURATION OF PROCEDURE: 4 hours.
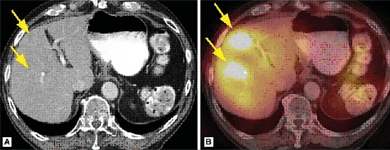
FUSION ONCOLOGIC SPET-CT
DESCRIPTION: Obtaining fusion images by combining the functional images of tomoscintigraphy or SPET with the anatomical images of CT in the study of oncological processes. In this way, greater accuracy is achieved in the location and characterization of the lesions detected by the planar or tomographic studies without fusing.
PREPARATION: Variable, according to tracer and pathology. Please ask us.
INDICATIONS: Localization of bone metastases due to lung, prostate, kidney, breast cancer and others (with diphosphonates). Localization of differential thyroid cancer (with 123I). Localization of lymphomas (with 67Ga). Localization of neuroendocrine tumors (with octreotide or MIBG). Localization of the sentinel lymph node (in breast cancer, head and neck cancer, melanoma, etc.).
DURATION OF PROCEDURE: Variable, according to tracer and pathology.
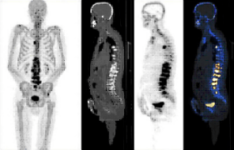
18F-SODIUM FLUORIDE (18F-NaF) PET-CT
DESCRIPTION: Study of bone metabolism.
PREPARATION: No previous preparation is needed. No fasting required.
INDICATIONS: Study of primary bone tumors, bone viability, bone necrosis and other benign bone disorders. Bone staging in cases of prostate and breast cancer and other neoplastic diseases with blastic bone metastases.
DURATION OF PROCEDURE: 1 hour.
abc

18F-DOPA PET-CT FOR THE PEDIATRIC
EVALUATION OF NEUROBLASTOMAS
AND OTHER NEUROENDOCRINE TUMORS
DESCRIPTION: Study of dopaminergic uptake in neuroblastomas and other TNEs in pediatric age.
PREPARATION: A 4-hour minimum fasting is required.
INDICATIONS: Evaluation of neuroblastomas and other TNEs.
DURATION OF PROCEDURE: 1 hour.
META-IODO-BENZYL-GUANIDINE (MIBG)
SCINTIGRAPHY
META-IODO-BENZYL-GUANIDINE (MIBG)
SCINTIGRAPHIC SCAN
META-IODO-BENZYL-GUANIDINE (MIBG)
TOMOSCINTIGRAPHY (SPET/SPET-CT)
DESCRIPTION
Evaluation of the existence of metabolic activity of the catecholamines pathway at the adrenal level and in neuroendocrine tumors.
PREPARATION
-
The thyroid gland must be stunned with 20 drops of potassium iodide 45-60 minutes before the I-123 tracer injection.
-
This preventive treatment must be started 5 days before the administration of the dose and must extend until 3 days after the end of the procedure if it is done with I-131.
INDICATIONS
Identification, location and evaluation of the extension of tumors of neuroectodermal tissue:
- Poisonous or malignant pheochromocytoma of intra or extra-adrenal localization
- Neuroblastomes
- Carcinoid tumors
- Medular thyroid carcinoma
- Paragangliomes
- Multiple endocrine neoplasies (MEN IIa and MEN IIb).
DURATION OF PROCEDURE
2 days.
ONCOLOGIC PET-CT WITH 18F-FDG
DESCRIPTION
Study of oncologic patients.
PREPARATION
-
Fasting of at least 6 hours before performing the procedure is required. In general, it is advisable that patients take a light meal the night before the study and that they do not ingest anything until after it has been done.
-
On the day of the procedure, the patient will have to drink abundant water (0.5-1L). Juices, sugary solutions, sweets etc. are not allowed. Urine must not be retained. Intravenous glucose solutions should also interrupt at least 6 hours before the scheduled time of the procedure.
-
The patient should take his/her usual medication.
-
It is necessary to avoid doing physical exercises 2-3 days before the procedure since this could condition a high muscular uptake of the tracer.
-
Diabetic type II and hyperglycemic patients treated with oral antidiabetic medicines should take their usual medication early in the morning and preferably stay in bed at night before performing the test.
-
Type I or diabetic patients with subcutaneous/intravenous insulin therapy should take their usual medication early in the morning. To avoid possible hypoglycemia, these patients can take some light breakfast, with a minimum of 4 hours before the procedure.
INDICATIONS
- Characterization of pancreatic masses.
- Detection of:
- Lung pulmonary nodules.
- Evident tumors of unknown origin.
- Detection of recurrences of:
- Gliomas with high degree of malignancy.
- Head and neck tumors.
- Differential thyroid cancer (high TG and negative RCI).
- Primary lung carcinoma.
- Breast carcinoma.
- Pancreatic carcinoma.
- Colorectal carcinoma.
- Ovarian cancer.
- Malignant lymphoma.
- Malignant melanoma.
- Other carcinomas.
- Staging of:
- Malignant melanoma.
- Locally advanced breast cancer.
- Head and neck tumors.
- Primary lung cancer.
- Aesophagus cancer.
- Pancreatic cancer.
- Colorectal cancer.
- Malignant lymphomas.
- Monitoring of the response to the treatment of:
- Malignant lymphomas
- Head and neck tumors.
DURATION OF PROCEDURE
2 to 3 hours.
SCAN FOR THE DETECTION OF
THYROID METASTASES
I-123 SCINTIGRAPHIC SCAN
(WITH OR WITHOUT rhTSH)
I-131 SCINTIGRAPHIC SCAN
(WITH OR WITHOUT rhTSH)
I-123 TOMOSCINTIGRAPHY
(SPET/SPET-CT)
DESCRIPTION
Evaluation of the metabolic activity of thyroid tissue remnants of differentiated thyroid neoplasms.
PREPARATION
-
The patient must suspend his/her hormonal treatment
-
Thyroxin [T4] for at least 21 days
-
Triyodothyronine [T3] for at least 10 days)
-
If hormonal suppression is not advised, TSH can be stimulated with two intramuscular injections in two consecutive days of rhTSH (Thyrogen).
-
The patient must not be in iodic saturation, either because of intravenous or intrathecal scans with iodized contrast (skinography, TAC with contrast, myelography and some angiographies) at least 3 weeks before the procedure, or because of treatment with amniodarone at least 6 months before the procedure.
INDICATIONS
-
Evaluation of the existence of functioning thyroid tissue remnants after a thyroidectomy.
-
Study of the spread of differentiated thyroid carcinoma.
-
Detection of recurrences of differentiated thyroid carcinoma.
DURATION OF PROCEDURE
3 days.
SCINTIGRAPHY WITH
SOMATOSTATIN RECEPTORS
SCINTIGRAPHY-SPECT-CT WITH 111In
OR 99mTc-OCTREOTIDE
SCINTIGRAPHIC SCAN WITH
SOMATOSTATIN RECEPTORS
TOMOGRAPHY-SPECT-CT WITH
SOMATOSTATIN RECEPTORS
DESCRIPTION
Evaluation of somatostatin receptor activity in tumors.
PREPARATION
- No scans with barium or iodine contrast should be performed during the procedure.
- For the study of abdominal tumors, it is advisable to use laxatives, and even the use of enemas.
INDICATIONS
- Evaluation of tumors that express somatostatin receptors (extension study, follow-up, control of therapeutic response and recurrence detection) in neuroendocrine tumors, such as carcinoid, enteropancreatic tumors and tumors derived from the neural crest (medullary of thyroid, neuroblastomas, paragangliomas and pheochromocytomas).
- Evaluation of other types of neoplasms that may have expression of somatostatin receptors, such as breast cancer, lung cancer, lymphomas and thyroid cancer.
DURATION OF PROCEDURE
1 to 2 days.
SCINTIGRAPHIC SCAN WITH TUMORAL AFFINITY
OR METABOLIC ACTIVITY TRACERS
(THALLIUM CHLORIDE, 99mTc-MIBI)
TOMOSCINTIGRAPHY (SPET/SPET-CT)
WITH TUMORAL AFFINITY OR METABOLIC
ACTIVITY TRACERS
DESCRIPTION
Evaluation of metabolic activity in tumors.
PREPARATION
No previous preparation is needed.
INDICATIONS
Evaluation of tumoral activity in general (pulmonary, muscular, breast, brain, etc.).
DURATION OF PROCEDURE
4 hours.
FUSION ONCOLOGIC SPET-CT
DESCRIPTION
Obtaining fusion images by combining the functional images of tomoscintigraphy or SPET with the anatomical images of CT in the study of oncological processes. In this way, greater accuracy is achieved in the location and characterization of the lesions detected by the planar or tomographic studies without fusing.
PREPARATION
Variable, according to tracer and pathology. Please, ask us.
INDICATIONS
- Localization of bone metastases due to lung, prostate, kidney, breast cancer and others (with diphosphonates).
- Localization of differential thyroid cancer (with I-123).
- Localization of lymphomas (with Ga-67).
- Localization of neuroendocrine tumors (with octreotide or MIBG).
- Localization of the sentinel lymph node (in breast cancer, head and neck cancer, melanoma, etc.).
DURATION OF PROCEDURE
Variable, according to tracer and pathology. Please, ask us.
18F-SODIUM FLUORIDE (18F-NaF) PET-CT
DESCRIPTION
Study of bone metabolism.
PREPARATION
- No previous preparation is needed.
- No fasting required.
INDICATIONS
- Study of:
- Primary bone tumors.
- Bone viability.
- Bone necrosis.
- Other benign bone disorders.
- Bone staging of:
- Prostate cancer.
- Breast cancer.
- Other neoplastic diseases with blastic bone metastases.
DURATION OF PROCEDURE
1 hour.
18F-DOPA PET-CT FOR THE PEDIATRIC
EVALUATION OF NEUROBLASTOMAS
AND OTHER NEUROENDOCRINE TUMORS
DESCRIPTION
Study of dopaminergic uptake in neuroblastomas and other TNEs in pediatric age.
PREPARATION
A 4-hour minimum fasting is required.
INDICATIONS
Evaluation of neuroblastomas and other TNEs.
DURATION OF PROCEDURE
1 hour.

SIMM MOLECULAR, S.L.U. belongs to the ATRYS Group
ATRYS-SIMM IRLA Center
Josep Irla i Bosch Street, 5, Buildong 2
Barcelona 08034, Catalonia, SPAIN
Phone (+34) 93 204 6439
ATRYS-SIMM SJD Center
Santa Rosa Street, 39, Pediatric Cancer Center Barcelona
Esplugues de Llobregat 08950, Catalonia, SPAIN
Phone (+34) 93 254 0470
FAX: (+34) 93 204 9641 · EMAIL: info.simm@atryshealth.com
In ATRYS-SIMM your personal data is treated according to the principles and rights included in the Spanish laws GDPR 2016/679 of April 27th, 2016 and LOPDGDD 3/2018 of December 5th, 2018.
You can excercise your rights by contacting us by email to protecciondatos-sp@atryshealth.com.
SIMM MOLECULAR, S.L.U. belons to the ATRYS Group
ATRYS-SIMM IRLA
Josep Irla i Bosch Street, 5, Building 2
Barcelona 08034, Catalonia, SPAIN
Phone (+34) 93 204 6439
ATRYS-SIMM SJD
Santa Rosa Street, 39
Esplugues de Llobregat 08950, Catalonia, SPAIN
Phone (+34) 93 254 0470
FAX: (+34) 93 204 9641
E-MAIL: info.simm@atryshealth.com
At ATRYS-SIMM your personal data is treated in accordance with
the principles and rights contained in the GDPR law 2016/679 of
April 27, 2016 and in the LOPDGDD law 3/2018 of December 5, 2018.
You can exercise your rights by contacting us by
email to protecciondatos-sp@atryshealth.com.