METABOLIC THERAPY PROCEDURES
It is mandatory to present your medical prescription to be able to carry out the tests.
If you are a pregnant woman or if you suspect that you may be so, or if you are breast-feeding,
please notify it to our health staff before performing any test.
All nuclear medicine tests involve the administration of a radiopharmaceutical
with a very low dose of radioactivity, which is eliminated in the following hours through the urinary or digestive routes.
The access of companions to the center is allowed in the case of children and patients who require special attention.
The rest of the patients can come accompanied but, as a health precaution during the pandemic and depending on
the capacity of the waiting room and the criteria of our staff, it is possible that
the companion cannot wait inside the center.
METABOLIC THERAPY PROCEDURES
It is mandatory to present your medical prescription to be able to carry out the tests.
If you are a pregnant woman or if you suspect that you may be so, or if you are breast-feeding,
please notify it to our health staff before performing any test.
All nuclear medicine tests involve the administration of a radiopharmaceutical
with a very low dose of radioactivity, which is eliminated in the following hours through the urinary or digestive routes.
The access of companions to the center is allowed in the case of children and patients who require special attention.
The rest of the patients can come accompanied but, as a health precaution during the pandemic and depending on
the capacity of the waiting room and the criteria of our staff, it is possible that
the companion cannot wait inside the center.
abc
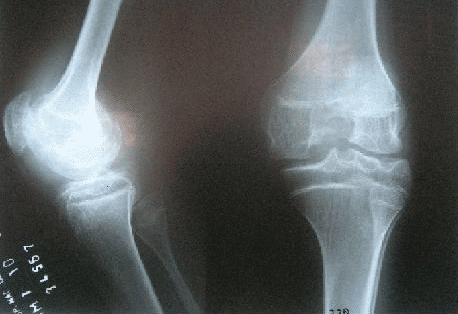
RADIOISOTOPE TREATMENT OF SYNOVITIS IN INFLAMATORY CHRONIC ARTHROPATHIES
ENDOCAVITARY RADIOISOTOPE TREATMENT
DESCRIPTION: Treatment of synovitis due to chronic inflammatory arthropathies.
PREPARATION: Treatment is done in agreement with the doctor who indicates it (rheumatologist, traumatologist, systemic illnesses specialist, etc.) and once he/she has established that there are no local contraindications (backed-up with X-rays, IRM or TC if necessary).
INDICATIONS: Treatment of synovitis due to chronic inflammatory arthropathies, such as rheumatoid arthritis and other arthropathies resistant to medical treatment (seronegative arthropathy, hemophilia, villonodular synovitis). Possibility of treating chronic degenerative arthropathy.
DURATION OF PROCEDURE: 30 minutes.

RADIOISOTOPE TREATMENT OF HYPERTHYROIDISM
DESCRIPTION: Treatment of hyperthyroidism with I-131.
PREPARATION: If the patient’s clinic allows it, the antithyroid treatment must be withdrawn 7 days before the treatment. In severe hyperthyroidism or in cases of cardiological involvement, it is not necessary to remove the antithyroid treatment. The β-blockers are maintained (on demand). A thyroid blood analysis (total T4 and T3, free T4, TSH and antithyroid antibodies) must be performed as well as routine blood count checks before administering the dose. A pregnancy test must be performed to fertile-age women, which must be negative. A control of therapeutic efficacy by whole-body scan and SPECT/CT must be performed on the fifth day after the treatment. Patients who do not reach euthyroidism without antithyroid treatment at 3 months will be assessed to receive a new dose of I-131 with the same protocol. In the case of recurrence of disease, the same protocol is followed.
INDICATIONS: Treatment of Basedow disease (diffuse goiter). Treatment of multinodular goiter. Treatment of toxic adenoma.
DURATION OF PROCEDURE: 1 hour.
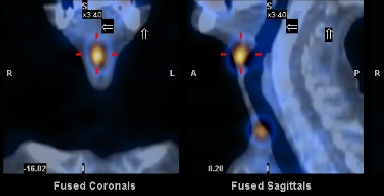
RADIOISOTOPE TREATMENT OF
DIFFERENTIATED LOW-RISC
THYROID CANCER
(REMNANTS ABLATION)
DESCRIPTION: Treatment of differentiated low-risk thyroid cancer with I-131.
PREPARATION: The antithyroid treatment must be withdrawn 4 weeks before the treatment or else, rhTSH stimulation must be done. A thyroid blood analysis (with basal and stimulated thyroglobulin measurements) and a regular blood count must be performed before administering the dose. A pregnancy test must also be done to fertile-age women, which must be negative. A control of therapeutic efficacy by whole-body scan and SPECT/CT must be performed on the fifth day after the treatment. Patients who do not achieve ablation can repeat the treatment 6 months later under the same protocol. In case of recurrence, the same protocol is followed.
INDICATIONS: Treatment of differentiated low-risk thyroid cancer.
DURATION OF PROCEDURE: 1 hour.
abc
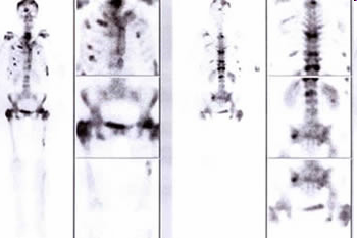
RADIOISOTOPE TREATMENT OF METASTATIC BONE PAIN WITH STRONTIUM-89 (METASTRON), SAMARIUM-153 (QUADRAMET) AND RADIUM-223 (XOFIGO)
DESCRIPTION: Treatment of pain produced by bone metastasis of prostate or breast carcinoma, resistant to hormone therapy, chemotherapy or analgesics
PREPARATION: No previous preparation is needed.
INDICATIONS: Treatment of bone pain produced by bone metastases of prostate or breast cancer resistant to chemo and hormone therapy or to analgesics. To receive treatment the patient must:
- Presence of pain caused by bone metastases, coinciding the location of the pain with images compatible with metastasis in a bone scintigraphy. The Karnofsky Index must be 60 or higher. Patients with lower numbers may receive treatment, although in these cases the response is not so favorable.
- Presence of pain despite regular treatment with opioid or non-opioid analgesics.
- Patient’s must have preservation of his/her renal function and a hematological function with platelets counts greater than 100 x 109/L and leukocytes counts greater than 3 x 109/L.
DURATION OF PROCEDURE: 15 minutes.
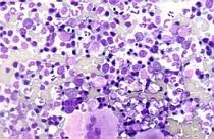
RADIOISOTOPE TREATMENT OF POLYCYTHEMIA VERA AND ESSENTIAL THROMBOCYTHEMIA
DESCRIPTION: Treatment of polycythemia vera and essential thrombocytosis with P-32 in the form of sodium phosphate.
PREPARATION: No preparation is required. Childbearing-age women with the possibility of being pregnant must not be subjected to this treatment.
INDICATIONS: Erythrocytosis not controlled by phlebotomies. Excessive thrombocytosis, with platelet counts over 800,000 per cubic mm. Extramedullary hematopoiesis with splenic pain, hypersplenicity, abdominal pain or splenic infarction. Cardiovascular pathology in which repeated phlebotomies are contraindicated. Persistent symptoms, such as pruritus or hyperuricemia not controlled by phlebotomy, antihistamines or allopurinol.
DURATION OF PROCEDURE: 15 minutes.
LYMPHOMA TREATMENT WITH ZEVALIN
DESCRIPTION: Treatment and induction to remission of follicular non-Hodgkin’s lymphoma of B CD20 + cells in relapse or refractories to treatment with Rituximab. Zevalin combines the targeted capability of an anti CD20 monoclonal antibody with the cytotoxic potential of yttrium-90 to destroy tumor cells (Ibritumomab, Tiuxetan).
PREPARATION: No special preparation needed. In patients with very low platelet counts, the dose should be reduced.
INDICATIONS: Follicular no-Hodgkin lymphoma of B CD20 + cells in relapse or refractory to treatment with Rituximab.
DURATION OF PROCEDURE: 4 hours.
abc
MEDICAL APPOINTMENT
DESCRIPTION: Visit with one of our Nuclear Medicine specialists.
PREPARATION: None. Previous medical reports and medication, if any, must be at hand.
INDICATIONS: Visit for patients receiving metabolic therapy for hyperthyroidism, bone pain, sinoviortesis or other specific reasons.

EMERGENCY MEDICAL APPOINTMENT
DESCRIPTION: Emergency visit with one of our Nuclear Medicine specialists.
PREPARATION: None. Previous medical reports and medication, if any, must be at hand.
INDICATIONS: Visit for patients receiving metabolic therapy for hyperthyroidism, bone pain, sinoviortesis or other specific reasons.
RADIOISOTOPE TREATMENT OF SYNOVITIS
IN INFLAMATORY CHRONIC ARTHROPATHIES
ENDOCAVITARY RADIOISOTOPE TREATMENT
DESCRIPTION
Treatment of synovitis due to chronic inflammatory arthropathies.
PREPARATION
Treatment is done in agreement with the doctor who indicates it (rheumatologist, traumatologist, systemic illnesses specialist, etc.) and once he/she has established that there are no local contraindications (backed-up with X-rays, IRM or TC if necessary).
INDICACIONES
-
Treatment of synovitis due to chronic inflammatory arthropathies.
-
Treatment of rheumatoid arthritis or other arthropathies resistant to medical treatment (seronegative arthropathy, hemophilia, villonodular synovitis).
-
Possibility of treating chronic degenerative arthropathy.
DURATION OF PROCEDURE
30 minutes.
RADIOISOTOPE TREATMENT OF HYPERTHYROIDISM
DESCRIPCIÓN
Treatment of hyperthyroidism with I-131.
PREPARATION
-
If the patient’s clinic allows it, the antithyroid treatment must be withdrawn 7 days before the treatment. In severe hyperthyroidism or in cases of cardiological involvement, it is not necessary to remove the antithyroid treatment.
-
The β-blockers are maintained (on demand).
-
A thyroid blood analysis (total T4 and T3, free T4, TSH and antithyroid antibodies) must be performed as well as routine blood count checks before administering the dose.
-
A pregnancy test must be performed to fertile-age women, which must be negative.
-
Therapeutic efficacy control by means of whole-body scan and SPECT/CT must be performed on the fifth day after treatment.
-
Patients who do not reach euthyroidism without antithyroid treatment at 3 months will be assessed to receive a new dose of I-131 with the same protocol. In the case of recurrence of disease, the same protocol is followed.
INDICATIONS
-
Treatment of Basedow disease (diffuse goiter).
-
Treatment of multinodular goiter.
-
Treatment of toxic adenoma.
DURACIÓN DEL PROCEDIMIENTO
1 hora.
RADIOISOTOPE TREATMENT OF
DIFFERENTIATED LOW-RISC
THYROID CANCER
(REMNANTS ABLATION)
DESCRIPTION
Treatment of differentiated low-risk thyroid cancer with I-131.
PREPARATION
- The antithyroid treatment must be withdrawn 4 weeks before the treatment or else, rhTSH stimulation must be done.
- A thyroid blood analysis (with basal and stimulated thyroglobulin measurements) and a regular blood count must be performed before administering the dose.
- A pregnancy test must also be done to fertile-age women, which must be negative.
- A control of therapeutic efficacy by whole-body scan and SPECT/CT must be performed on the fifth day after the treatment.
- Patients who do not achieve ablation can repeat the treatment 6 months later under the same protocol.
- In case of recurrence, the same protocol is followed.
INDICATIONS
Treatment of differentiated low-risk thyroid cancer.
DURATION OF PROCEDURE
1 hour.
RADIOISOTOPE TREATMENT OF
METASTATIC BONE PAIN WITH
STRONTIUM-89 (METASTRON),
SAMARIUM-153 (QUADRAMET)
AND RADIUM-223 (XOFIGO)
DESCRIPTION
Treatment of pain produced by bone metastasis of prostate or breast carcinoma, resistant to hormone therapy, chemotherapy or analgesics.
PREPARATION
No previous preparation is needed.
INDICATIONS
Treatment of bone pain produced by bone metastases of prostate or breast cancer resistant to chemo and hormone therapy or to analgesics. To receive treatment the patient must:
- Presence of pain caused by bone metastases, coinciding the location of the pain with images compatible with metastasis in a bone scintigraphy. The Karnofsky Index must be 60 or higher. Patients with lower numbers may receive treatment, although in these cases the response is not so favorable.
- Presence of pain despite regular treatment with opioid or non-opioid analgesics.
- Patient’s must have preservation of his/her renal function and a hematological function with platelets counts greater than 100 x 109/L and leukocytes counts greater than 3 x 109/L.
DURATION OF PROCEDURE
15 minutes.
RADIOISOTOPE TREATMENT OF
POLYCYTHEMIA VERA AND
ESSENTIAL THROMBOCYTHEMIA
DESCRIPTION
Treatment of polycythemia vera and essential thrombocytosis with P-32 in the form of sodium phosphate.
PREPARATION
-
No preparation is required.
-
Childbearing-age women with the possibility of being pregnant must not be subjected to this treatment.
INDICATIONS
-
Erythrocytosis not controlled by phlebotomies.
-
Excessive thrombocytosis, with platelet counts over 800,000 per cubic mm.
-
Extramedullary hematopoiesis with splenic pain, hypersplenicity, abdominal pain or splenic infarction.
-
Cardiovascular pathology in which repeated phlebotomies are contraindicated.
-
Persistent symptoms, such as pruritus or hyperuricemia not controlled by phlebotomy, antihistamines or allopurinol.
DURATION OF PROCEDURE
15 minutes.
LYMPHOMA TREATMENT WITH ZEVALIN
DESCRIPTION
Treatment and induction to remission of follicular non-Hodgkin’s lymphoma of B CD20 + cells in relapse or refractories to treatment with Rituximab. Zevalin combines the targeted capability of an anti CD20 monoclonal antibody with the cytotoxic potential of yttrium-90 to destroy tumor cells (Ibritumomab, Tiuxetan).
PREPARACIÓN
-
No special preparation needed.
-
In patients with very low platelet counts, the dose should be reduced.
INDICATIONS
Follicular no-Hodgkin lymphoma of B CD20 + cells in relapse or refractory to treatment with Rituximab.
DURATION OF PROCEDURE
4 hours.
MEDICAL APPOINTMENT
DESCRIPTION
Visit with one of our Nuclear Medicine specialists.
PREPARATION
- None.
- Previous medical reports and medication, if any, must be at hand.
INDICATIONS
Visit for patients receiving metabolic therapy for:
- Hyperthyroidism
- Bone pain
- Sinoviortesis
- Other specific reasons
EMERGENCY MEDICAL APPOINTMENT
DESCRIPTION
Emergency visit with one of our Nuclear Medicine specialists.
PREPARATION
- None.
- Previous medical reports and medication, if any, must be at hand.
INDICATIONS
Visit for patients receiving metabolic therapy for:
- Hyperthyroidism
- Bone pain
- Sinoviortesis
- Other specific reasons

ATRYS-SIMM IRLA
Josep Irla i Bosch Street, 5, Building 2
Barcelona 08034, Catalonia, SPAIN
Phone (+34) 93 204 6439
ATRYS-SIMM SJD
Santa Rosa Street 39, Pediatric Cancer Center Barcelona
Esplugues de Llobregat 08950, Catalonia, SPAIN
Phone (+34) 93 254 0470
FAX: (+34) 93 204 9641 · E-MAIL: info.simm@atryshealth.com
At ATRYS-SIMM your personal data is treated in accordance with the principles and rights contained in the GDPR law 2016/679 of April 27, 2016 and in the LOPDGDD law 3/2018 of December 5, 2018.
You can exercise your rights by contacting us by email to protecciondatos-sp@atryshealth.com.
ATRYS-SIMM IRLA
Josep Irla i Bosch Street, 5, Building 2
Barcelona 08034, Catalonia, SPAIN
Phone (+34) 93 204 6439
ATRYS-SIMM SJD
Santa Rosa Street, 39
Esplugues de Llobregat 08950, Catalonia, SPAIN
Phone (+34) 93 254 0470
FAX: (+34) 93 204 9641
E-MAIL: info.simm@atryshealth.com
At ATRYS-SIMM your personal data is treated in accordance with
the principles and rights contained in the GDPR law 2016/679 of
April 27, 2016 and in the LOPDGDD law 3/2018 of December 5, 2018.
You can exercise your rights by contacting us by
email to protecciondatos-sp@atryshealth.com.


